The essay on hydrochloric acid and marble reactions.
Marble chips and hydrochloric acid experiment graph.
9 3 sketch a line on the grid in figure 10 to show the results you would expect if the experiment was repeated using 20 g of smaller marble chips.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Label this line a.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Volume of gas cm 3 on the vertical axis.
The reaction using a higher concentration.
Draw a curve of best fit.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
The graph which is most likely to be produced from experiment 3 is graph a the only difference in the conditions compared with experiment 1 is that there is twice as much acid.
Repeat the experiment with 20g of the same marble chip size and 20cm 3 of the acid made up to 40 using distilled water.
The graph which is most likely to.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
An investigation of the reaction between marble chips and hydrochloric acid.
For each concentration of hydrochloric acid plot a graph to show.
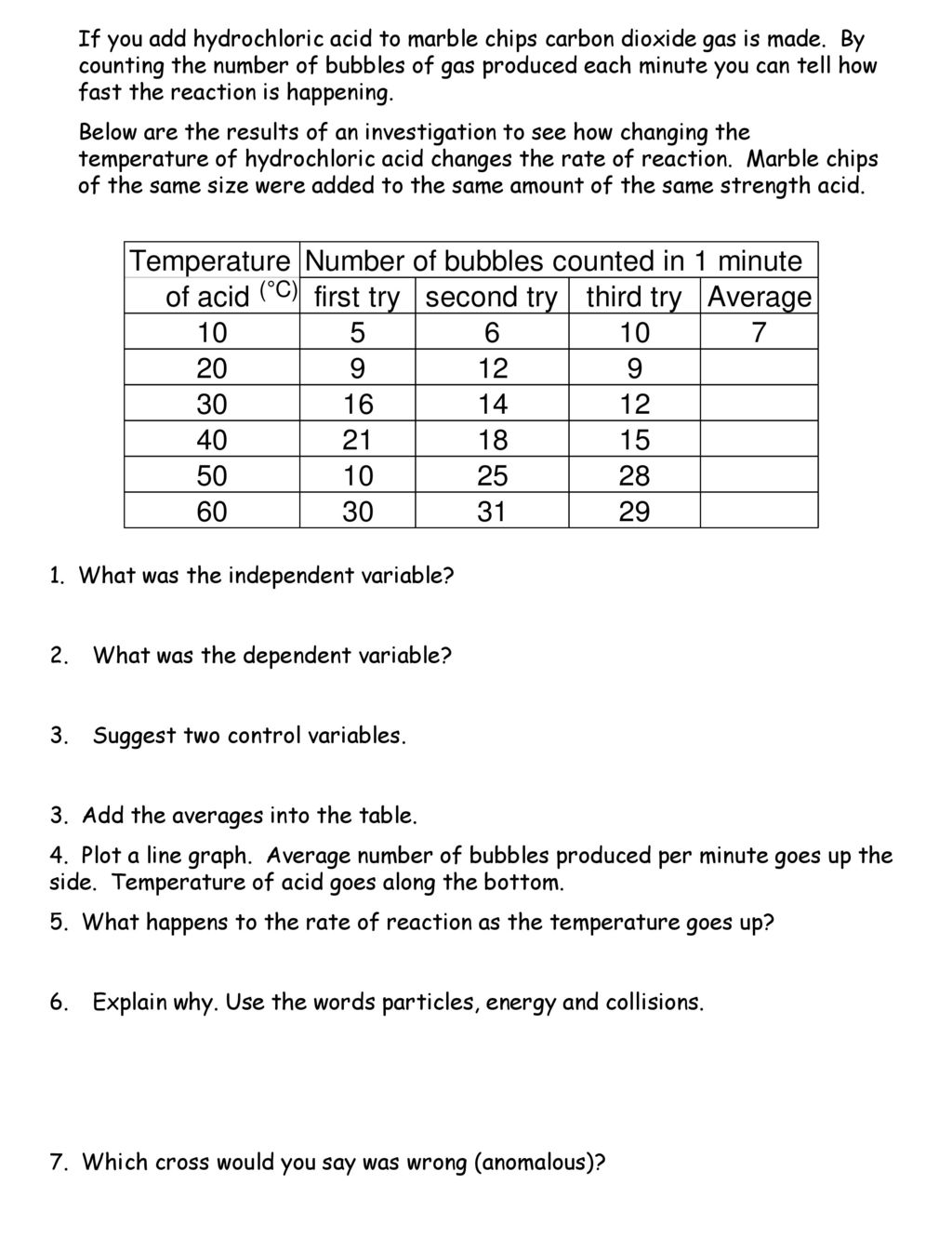
Dgfhhdhdinvestigating rates of reaction aim the aim of my investigation is to find out how rates of reaction are affected by temperature specifically how the rate of the reaction between hydrochloric acid and marble chips is affected by the temperature of the hydrochloric acid.
2 marks 9 4 explain in terms of particles how and why the rate of reaction changes during the reaction of calcium carbonate with hydrochloric acid.
The rate of this reaction can be changed by changing the size of the marble chips.
Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
This will not make any difference to the graph as it is the marble chips that are used up not the acid so the graph will be the same.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
Compare the slopes of the two graphs.
Time s on the horizontal axis.
There are many variables that affect.