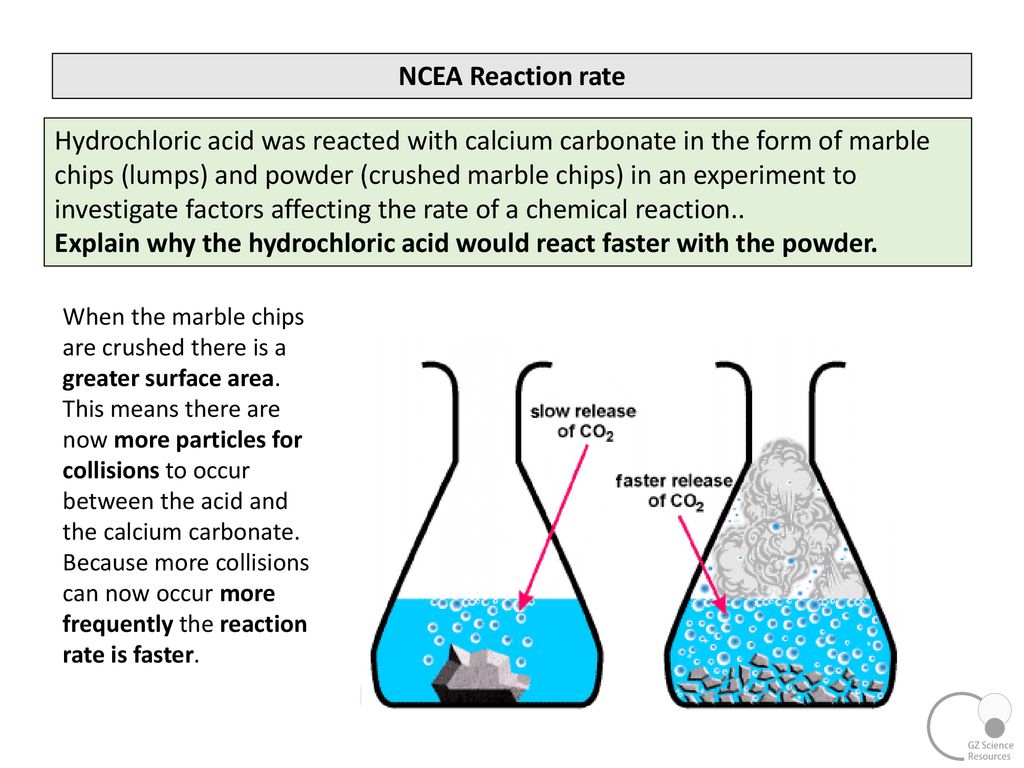
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
Marble chips and hydrochloric acid experiment surface area method.
Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
Hcl calcium carbonate calcium chloride carbon dioxide water.
Changing factors independent variables.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
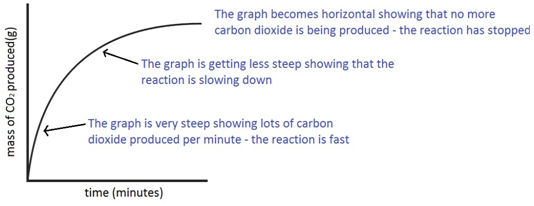
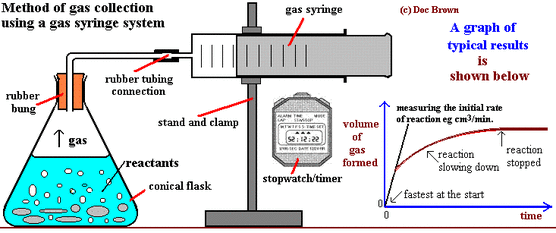
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
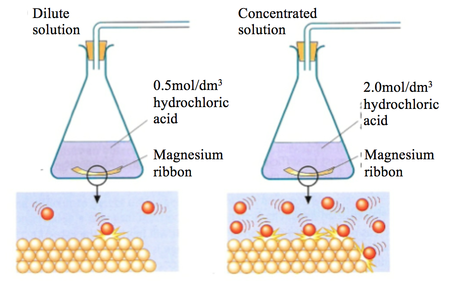
Add different concentrations of hydrochloric acid method.
As the marble chips react with.
And dilute hydrochloric acid calcium carbonate may be used in the form of marble chips.
Add hydrochloric acid into a conical flask.
2 to investigate the effect of temperature of sodium thiosulphate in a reaction with hydrochloric acid.
Finding the rate of reaction of marble chips and hydrochloric acid changing the surface area.
The surface area isn t always the same so even though the mass of the marble.
To investigate the effect of concentration of sodium thiosulphate in a reaction with hydrochloric acid.
There are many variables that affect.
Equipment list the equipment i will be using for this experiment to see how the surface area of marble chips caco3 affect the rate of reaction when placed in hydrochloric acid are 25 ml of hydrochloric acid 3g of marble chips graduated cylinder a stop clock test tube 250 ml beaker delivery tube scale ceramic mount and a plastic dish.
To observe the effect of surface area particle size on the rate of reaction between marble chips calcium carbonate and hcl.
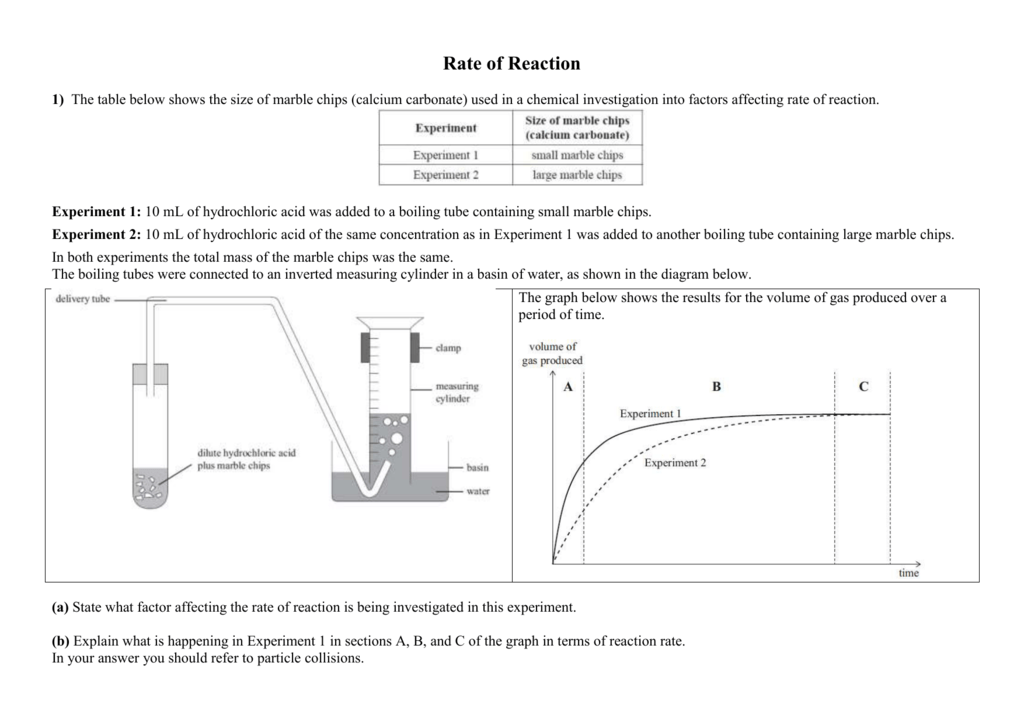
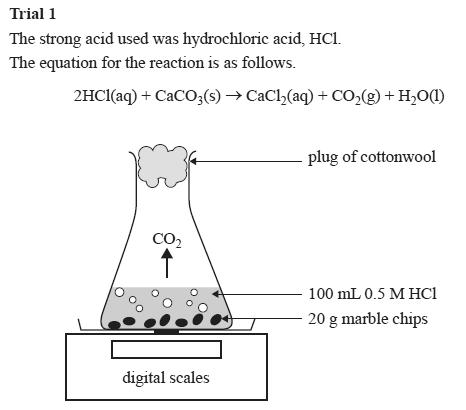
2hcl aq caco 3 s cacl 2 aq co 2 g h 2 o l the reaction rates of both large marble chips and small marble chips can be compared see below.
Calcium chloride solution is also formed.
I will weigh out one gram of marble chips using a balance and put it in a conical flask and add to it a concentration of 50cm3 using water and hydrochloric acid.
An investigation of the reaction between marble chips and hydrochloric acid.
Smaller chips have a larger surface area.
Use a capillary tube to connect this flask to a measuring cylinder upside down in a bucket of water downwards displacement.