This process is based on random particle movement.
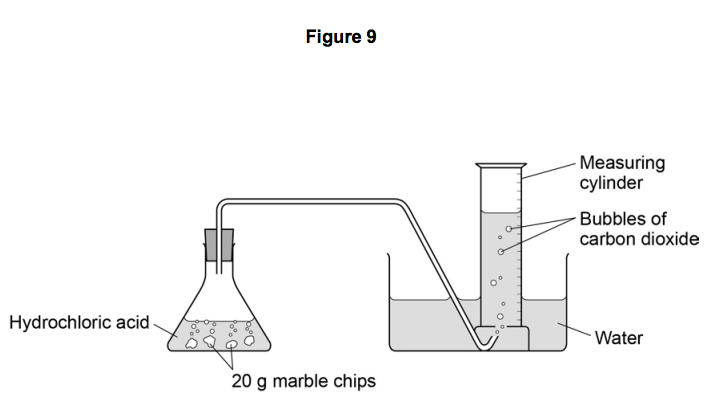
Marble chips and hydrochloric acid.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
There are many variables that affect.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
The rate of this reaction can be changed by changing the size of the marble chips.
Marble chips and hydrochloric acid planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.